Copyright 2011© by Laszlo Zopcsak PhD
The inclusion of resistance training (RT) as a part of an exercise therapy program has been endorsed by the American Heart Association [64], the American College of Sports Medicine [65, 66], and the American Diabetes Association [67].While these recommendations are primarily based on the effects of RT on muscle strength, cross-sectional studies have shown that muscle mass is inversely associated with all-cause mortality [68] and the prevalence of the metabolic syndrome [69, 70], independent of cardiorespiratory fitness levels. Evidence suggests that skeletal muscle is responsible for up to 40% of individuals’ total body weight and may be influential in modifying metabolic risk factors via muscle mass development. Due to the metabolic consequences of reduced muscle mass, it is understood that normal aging and/or decreased physical activity may lead to a higher prevalence of metabolic disorders. Overall, strong evidence supports that regular RT can effectively alter body composition in obese, previously sedentary men and women, independently from dietary restriction. It has been shown that RT increases LBM, muscular strength, and resting metabolic rate, and mobilizes the visceral and subcutaneous adipose tissue in the abdominal region [71].
Numerous studies suggest that strength training may be an effective means of rebuilding muscle, recharging metabolism, and reducing fat in previously inactive adults, as summarized in Table 2.2.
Table 2.3. Health Benefits of Resistance Training (Taken from Westcott, W.L., ACSM Strength Training Guidelines: Role in Body Composition and Health Enhancement. ACSM's Health & Fitness Journal, 2009. 13(4): p. 14-22. [70]
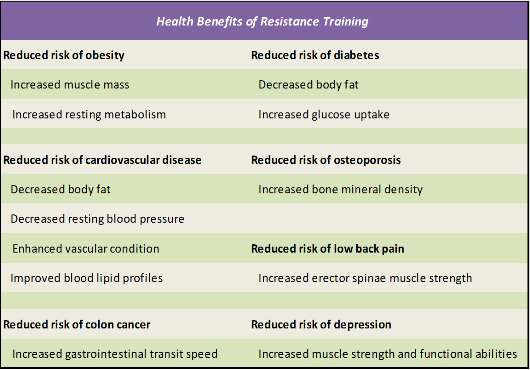
Information obtained from references [72-79]
2.2.1. Changes of Absolute and Relative Strength, Adaptation to RT
At first glance when designing a periodized RT program for women, it seems that there is a huge difference between a female program and one designed for males. It has been commonly believed for a long time that women's adaptations to RT are less than men's, and therefore that women would benefit less from RT than men [80]. But in reality there are very few differences in these program designs. Female skeletal muscle responds to a resistance programs with similar adaptations as males and research to date, indicates that RT is at least as advantageous, for women as for men, if not more so [4, 12].
The main goal of an RT program can be body compositional changes for women as well as for men. Increases in lean body mass and decreases in percent body fat from short-term (8 to 20 week) RT programs are of the same degree in women as they are in men. Muscle fiber hypertrophy can occur in women who perform RT [81]. The transition of the myosin heavy chains starts to take place within a couple of workouts in women faster than that observed in men [22]. It seems that body composition and fiber type changes from RT take place equally in both genders or possibly faster in women. Using identical RT programs, women gain strength at the same rate or faster than men [12, 82, 83]. Studies show that men have greater absolute increases, but relative % increases can be the same or greater in women than in men. Commonly, adult males are stronger than adult females. The reason for this can be that adult males are usually larger and have greater fat free mass than females. Indeed, some studies point to the fact that females are stronger than males when lower body maximal strength is articulated relative to fat free mass in their lower bodies than their male counterparts [21]. The average woman's maximal mean total body strength is 63.5% of the average man's; women's isometric upper-body strength averages 55.8% of men's; women's isometric lower-body strength averages 71.9% of men's [84].
Wilmore reported that women's 1RM bench press is 37% that of men [82]. If we express the bench press relative to body weight or lean body mass, women are 46% and 55%, as strong as men, respectively. Women's maximal isometric force during leg press is 73% that of men. If leg press strength is expressed relative to body weight and lean body mass, women are 92% and 106% as strong as men. In this respect strength differences between the genders are seen to be greatly reduced.
As it was described in the previous chapter, the level of muscle strength plays an important factor in the prevention of osteoarthritis, osteoporosis, and body mass density. Specific modes and intensities of physical activity can preserve or increase skeletal muscle mass, strength, power, and intrinsic neuromuscular activation. Such effects appear to be similar in women and men and pervasive throughout their lifespan. These effects of progressive resistance training in young healthy men and women have been well described [85]. As reviewed by Kraemer and colleagues, high-intensity progressive resistance training in young adults results in significant increases in dynamic strength, explosive power, and muscle mass.
Resistance training also has benefits in weight control. In a 2-year study of resistance training in overweight and obese premenopausal women, Schmitz and colleagues [86] reported a 4% decrease in total fat in the exercise group versus a negligible change in the control group (P<0.01). Interestingly, intra-abdominal fat increased over 2 years by 7% in the exercise group and by 21% in the control group, underscoring the benefits of resistance training (without caloric restriction) in minimizing intra-abdominal fat gain in middle-aged women. The benefits of resistance training may be most noticeable among obese and older populations, who typically have the greatest amount of abdominal fat. Nonetheless, Ross and colleagues [41] report that, even without coincident weight loss, 60 minutes per day of vigorous-intensity exercise (approximately 500 kilocalories per day) on 7 days per week still resulted in statistically significant reductions in total (7%), abdominal (10%), and intra-abdominal (18%) fat in abdominally-obese premenopausal women.
2.2.2. Muscle Power
Muscle power (the rate at which muscle force can be generated) is often associated with functional fitness, especially from the important aspect of reducing the onset of reduced ability and the prevention of falls in older adults. This population group has the highest prevalence of disability and falls [87] of any age group. Studies consistently show that the prevalence of both major disability and falls increases with age, and it is higher in women than in men [6]. It is of paramount importance that functional strength and power development start during the premenopausal stage of woman. Muscle power output may be a critical determinant of physical functioning in the elderly, and evidence is emerging that resistance training performed at high velocity and low external resistance to maximize muscle power output may have important beneficial effects on physical function in older adults [6].
Although physical activity interventions that increase or maintain muscle strength have important health implications, emerging evidence suggests that muscle power may play a more important role in functional independence in any age. Muscle power has been shown to decline more precipitously with aging than does dynamic and isometric strength [88]. Lower extremity muscle power is also a strong predictor of physical performance, functional mobility, and reducing the risk of falling among older adults. Muscle power has been found to be inversely associated with self-reported disability status in community-dwelling older adults with mobility limitations [89] and is a better discriminator of mobility limitations than muscle strength [90].
Most trials that have evaluated the effects of progressive resistance training on muscle strength and mass have traditionally involved relatively slow movement velocities. Some of these have examined changes in lower extremity power output but more recently Delmonico and colleagues examined the effects of moderate-velocity resistance training on changes in peak power in older men and women [91]. They observed similar changes in absolute peak power in response to 10 weeks of resistance training in both older men and women. However, the relative improvements in peak power were greater in women (16%) compared to the men (11%).
Similar results have also been reported by Newton and colleagues employing a "periodized" resistance training intervention in healthy young and older men [92]. These studies suggest that traditional slow-velocity resistance training results in minimal improvements in peak power, that adaptations may be sex-dependent, and that resistance training performed at relatively slow velocities may lack the specificity to improve peak power, particularly in older individuals. Randomized trials that examined high-velocity resistance training to increase muscle power in older subjects compared the effects against slow velocity resistance training [93]. Fielding and colleagues compared high-velocity lower-extremity resistance training with traditional slow-velocity resistance training in older women with self-reported disability and observed an 84% greater increase in leg press power in the high-velocity training group. [94]. On the other hand women generate less power per unit volume of muscle than men. Although the data is not consistent it appears that the skeletal muscle's rate of force development is slower for the average woman than for the average man [95, 96], and other data indicates that women's power output relative to total body weight is 65% of men's [97].
In general, these studies all demonstrate that interventions designed to maximize muscle power are feasible, well tolerated, and can dramatically improve lower-extremity muscle power in healthy older men and women and with older women with self-reported disability.
2.2.3. Special Concerns about Women RT
A common misconception is that women's RT programs should be different than men's. Actually, the upper body shows the greatest difference in strength between the genders. This is because women tend to have a lower proportion of their lean body mass in their upper bodies, as previously discussed. [98]. The physiological characteristics of muscle are the same in both genders, and therefore respond to training in the same manner. Fiber composition may vary from muscle to muscle but in general both genders have the same percentage of Type I and II fibers in a particular muscle [99]. Women muscle has a greater amount of fat between the fascicles than male muscle. The amount of fat within the muscle is the main difference between male and female muscle, but this does not affect the trainability of muscle tissue.
Most women are afraid to implement heavy RT into their routine, because they believe that their muscles will hypertrophy and that they will look less feminine. However, in reality the average woman's muscles do not hypertrophy to an extreme, which is why it is not easy for woman body-builders to increase muscle size. On the other hand, muscle mass increase accompanied by a decrease in adipose tissue will result in no change or a slight decrease in body circumference, because muscle tissue is denser than adipose tissue [100]. Short-term studies of both lower- and upper-extremity resistance training have demonstrated increases in muscle cross-sectional area (CSA) in women [101], with corresponding increases in muscle strength. Sex-specific changes in muscle mass or CSA in response to resistance exercise training have been investigated. Short-term studies of progressive resistance training noted similar increases in muscle adaptations of men and women [22]. Increases in muscle CSA by computed tomography (CT) have also been shown to be similar in men (17.5%) and women (20.4%) in response to 16 week of upper- and lower-extremity high-intensity resistance training[12].
The greatest increase in various body circumferences in women from 10 week [82], 12 week [102], or 20 week RT [81] of framing was 0.6, 0.4, and 0.6 cm, respectively. These changes in circumference are practically unnoticeable. With a 10 week program hip, thigh and abdomen circumferences actually decreased 0.2 to 0.7 cm. Other studies also support the findings that RT in women results in small or no change in body circumferences [5]. Skinfold thickness also decreased during the 10-, 12-, and 16-week training studies discussed earlier. The lack of increases or even small decreases in body circumference is favorable for those women who want increased strength as well as a firm look of trained muscle without increased body circumferences. However, in contrast in Kok’s recent study that compared LP and UP periodization, muscle cross-sectional area (CSA) for both group significant increments were recorded. Pooled CSA increase was higher than previously found, and muscle hypertrophic responses were larger and occurred earlier than previously reported [11]. Few women develop a large amount of hypertrophy from RT due to individual factors such as higher than normal hormone levels, greater hormonal response to RT, lower than normal estrogen to testosterone ratio, genetic disposition to develop larger muscle mass, ability to perform more intense RT program than average women [103]. After a 6-month RT program increases of 3.5, 1.1, and 0.9 cm (5%, 4%, and 2%) were observed in a group of female athletes in shoulder girth, upper-arm circumference, and thigh circumference, respectively [82].
2.2.4. Endocrine System Adaptation
Physical activity in women can help to increase bone density. On the other hand menstrual dysfunction can lead to increased risk of osteoporosis and decreases in bone density [104-106]. The hormonal mechanisms that control menstrual cycle irregularities and bone loss are not completely explained, but it seems that physical stress from training, mental stress, and dietary deficiencies act through a common pathway [107]. The change of the concentrations of the ovarian hormones of estrogen and progesterone are the hormonal factors most frequently associated with osteoporosis and bone loss. Growth hormone, testosterone, estradiol, insulin, and calcitonin, can effect menstrual cycle disturbances and bone loss in active women [105, 107]. A major benefit of an exercise program is a decrease in normal premenstrual symptoms such as breast enlargement, appetite cravings, bloating, and mood changes. Women who have an active lifestyle and exercise on a daily basis have fewer problems with premenstrual symptoms than inactive women. However, premenstrual symptoms may increase, if training is decreased, especially if weight gain is concurrent with the decrease in training [107]. Female athletes should carefully plan training decrease, if they experienced excessive premenstrual symptoms earlier in their life. Training should not be decreased abruptly and large weight gains should be avoided. Likewise premenstrual symptoms, dysmenorrhea occurs less frequently and is less severe in active women than in the sedentary population [108]. Oligomenorrhea and secondary amenorrhea are more common in women whose training routine is demanding [107, 109, 110]. According to some limited data it seems that women engaged in RT may be at a greater risk than normal of acquiring menstrual cycle irregularities. Greater RT intensity or volume results in greater risk of menstrual irregularities. 33% of female body-building competitors - not taking oral contraceptives - reported oligomenorrhea or secondary amenorrhea [111]. However, not all athletes performing vigorous RT will experience menstrual irregularities.
At rest, men normally have 10 times more testosterone concentrations than women [112]. This may cause a larger increases in muscle hypertrophy in men than in women. Resting serum testosterone concentration remained unchanged in women during an 8-wk RT program [113]. However, it was reported in a 16-wk RT program, that although resting serum testosterone concentrations did not change significantly, individual mean serum levels of both total and free testosterone correlated significantly with changes in muscle force production in women [114]. The serum testosterone concentrations did not change significantly during one RT session in women's case, whereas men's serum testosterone concentrations did increase significantly from the same RT session [115-117]. However, the serum growth hormone increased significantly from the same RT session in both women's and men's case [117].
In summary RT performed by women can result in desirable fitness characteristics such as a fit appearance and increased strength and power for recreational and sport activities as well as quality life. However, there should be some caution that strenuous resistance activity may induce menstrual irregularities, compared to the sedentary normal population, and irregularities of the menstrual cycle normally cease once demanding RT is stopped. The beliefs that women’s muscle excessively hypertrophy through RT programs are unfounded. The magnitude of the adaptations to a RT program for women is the same or even better than men's concerning relative strength. RT programs for women do not have to be different than those for men, except that the absolute resistance used should normally be less.
REFERENCES
66. Pescatello, L.S., et al., American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc, 2004. 36(3): p. 533-53.
67. Sigal, R.J., et al., Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care, 2006. 29(6): p. 1433-8.
68. Fitzgerald, S.J. and B. G.S, Muscular fitness and all-cause mortality: prospective observations. Journal of Physical Activity and Health, 2004. 1: p. 17-18.
69. Zanuso, S., et al., Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol, 2010. 47(1): p. 15-22.
70. Westcott, W.L., ACSM Strength Training Guidelines: Role in Body Composition and Health Enhancement. ACSM's Health & Fitness Journal, 2009. 13(4): p. 14-22.
71. Strasser, B. and W. Schobersberger, Review Article: Evidence for Resistance Training as a Treatment Therapy in Obesity. Journal of Obesity, 2010. 2011: p. 9.
72. Boyden, T.W., et al., Resistance exercise training is associated with decreases in serum low-density lipoprotein cholesterol levels in premenopausal women. Arch Intern Med, 1993. 153(1): p. 97-100.
73. Braith, R.W. and K.J. Stewart, Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation, 2006. 113(22): p. 2642-50.
74. Gettman, L.R., P. Ward, and R.D. Hagan, A comparison of combined running and weight training with circuit weight training. Med Sci Sports Exerc, 1982. 14(3): p. 229-34.
75. Hoff, J., et al., Maximal strength training of the legs in COPD: a therapy for mechanical inefficiency. Med Sci Sports Exerc, 2007. 39(2): p. 220-6.
76. Hurley, B.F. and S.M. Roth, Strength training in the elderly: effects on risk factors for age-related diseases. Sports Med, 2000. 30(4): p. 249-68.
77. Jurca, R., LaMonte MJ, and e.a. Church TS, Associations with muscle strength and aerobic fitness with metabolic syndrome in men. Med Sci Sports Exerc, 2004. 36(8).
78. Koffler, K.H., et al., Strength training accelerates gastrointestinal transit in middle-aged and older men. Med Sci Sports Exerc, 1992. 24(4): p. 415-9.
79. Mason, C., et al., Musculoskeletal fitness and weight gain in Canada. Med Sci Sports Exerc, 2007. 39(1): p. 38-43.
80. Wells, C.L., ed. The female athlete: myths and superstitions put to rest. In toward an understanding of human performance. ed. E.J. Burke. 1978, Movement Press: Ithaca, NY.
81. Staron, R.S., et al., Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol, 1991. 70(2): p. 631-40.
82. Wilmore, J.H., Alterations in strength, body composition and anthropometric measurements consequent to a 10-week weight training program. Med Sci Sports, 1974. 6(2): p. 133-8.
83. Wilmore, J.H., et al., Physiological alterations consequent to circuit weight training. Med Sci Sports, 1978. 10(2): p. 79-84.
84. Laubach, L.L., Comparative muscular strength of men and women: a review of the literature. Aviat Space Environ Med, 1976. 47(5): p. 534-42.
85. Kraemer, W., Fleck SJ, and E. WJ., Strength and power training: physiological mechanisms of adaptation. Exerc.Sport Sci.Rev, 1996. 24: p. 363-97.
86. Schmitz, K., et al., Strength training and adiposity in premenopausal women: strong, healthy, and empowered study. Am.J.Clin.Nutr, 2007. 86(3): p. 566-72.
87. Campbell, A., Borrie MJ, and S. GF., Risk factors for falls in a community-based prospective study of people 70 years and older. J.Gerontol, 1989 44(4): p. M112-M117.
88. Metter, E., et al., Age-associated loss of power and strength in the upper extremities in women and men. J.Gerontol.A.Biol.Sci.Med.Sci. , 1997. 52(5): p. B267-B276.
89. Foldvari, M., et al., Association of muscle power with functional status in community-dwelling elderly women. J.Gerontol.A.Biol.Sci.Med.Sci, 2000. 55(4): p. M192-M199.
90. Bean, J., et al., A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J.Gerontol.A.Biol.Sci.Med.Sci, 2003. 58(8): p. 728-33.
91. Delmonico, M., et al., Effects of moderate-velocity strength training on peak muscle power and movement velocity: do women respond differently than men? . J.Appl.Physiol, 2005. 99(5): p. 1712-8.
92. Newton, R., et al., Mixed-methods resistance training increases power and strength of young and older men. Med.Sci.Sports Exerc, 2002. 34(8): p. 1367-75.
93. Fielding, R., et al., High-velocity resistance training increases skeletal muscle peak power in older women. J.Am.Geriatr.Soc, 2002. 50(4): p. 655-62.
94. Fielding, R.A., et al., High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc, 2002. 50(4): p. 655-62.
95. Komi, P.V. and J. Karlsson, Skeletal muscle fibre types, enzyme activities and physical performance in young males and females. Acta Physiol Scand, 1978. 103(2): p. 210-8.
96. Ryusi, T. and K. Hakkinen, Muscle fibre characteristics, muscle cross-sectional area and force production in strength athletes, physically active males and females. Scandinavian Journal of Sports Science, 1988. 10: p. 7-15.
97. Garhammer, J., ed. Weight lifting and training. Biomechanics of sport, ed. C.L. Vaughan. 1989, CRC Press: Boca Raton FL. 169-211.
98. Miller, A.E.J., J.D. MacDougall, and M.A. Tarnoplosky, Gender differences in strength and muscle fiber characteristics. European Journal Applied Physiology, 1992. 66: p. 254-62.
99. Drinkwater, B.L., Women and exercise: physiological aspects. Exerc Sport Sci Rev, 1984. 12: p. 21-51.
100. Mayhew, J.L. and P.M. Gross, Body composition changes in young women with high intensitiy of weight training. Research Quarterly, 1974. 45: p. 433-40.
101. Hisaeda, H., et al., Influence of two different modes of resistance training in female subjects. Ergonomics, 1996. 39(6): p. 842-52.
102. Boyer, B.T., A comaprison of the effects of three strength training programs on women. Journal of Applied Sport Sciences Research, 1990. 4: p. 88-94.
103. Fleck, S.J. and W.J. Kraemer, eds. Designing Resistance Training Programs. 3rd ed ed. 2004, Human Kinetics: Champaign, IL.
104. De Cree, C., A. Vermeulen, and M. Ostyn, Are high-performance young women athletes doomed to become low-performance old wives? A reconsideration of the increased risk of osteoporosis in amenorrheic women. J Sports Med Phys Fitness, 1991. 31(1): p. 108-14.
105. Cameron, K.R., J.D. Wark, and R.D. Telford, Stress fractures and bone loss - the sceletal cost of intense althleticism? Excel, 1992. 8: p. 39-55.
106. Nyburgh, K.H., et al., Low bone mineral density at axial and appendicular sites in amenorrheic athletes. Medicine and Science in Sports and Exercise, 1993. 25: p. 1197-1202.
107. Prior, J.C., Y.M. Vigna, and D.W. McKay, Reproduction for the athletic woman. New understandings of physiology and management. Sports Med, 1992. 14(3): p. 190-9.
108. Dale, E., D.H. Gerlach, and A.L. Wilhite, Menstrual dysfunction in distance runners. Obstet Gynecol, 1979. 54(1): p. 47-53.
109. Loucks, M.E., J.L. Morrill, and A.D. Dayton, Effect of prepartum vaccination with K99 Escherichia coli vaccine on maternal and calf blood antibody concentration and calf health. J Dairy Sci, 1985. 68(7): p. 1841-7.
110. DeSouza , M.J. and D.A. Metzger, Reproductive dysfunction in amennorrheic athletes and anorexic patients: A review. Medicine and Science in Sports and Exercise, 1991. 23: p. 995-1007.
111. Elliot, D.L. and L. Goldberg, Weight lifting and amenorrhea. JAMA, 1983. 249(3): p. 354.
112. Wright, J.E., Anabolic steroids and athletics. Exerc Sport Sci Rev, 1980. 8: p. 149-202.
113. Hetrick, G.A. and J.H. Wilmore, Androgen levels and muscle hypertophy during eight week weight training program for men/women. Medicine and Science in Sports and Exercise, 1979. 11: p. 102.
114. Hakkinen, K., et al., Neuromuscular adaptations and serum hormones in females during prolonged power training. Int J Sports Med, 1990. 11(2): p. 91-8.
115. Kraemer, W.J., et al., Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol, 1990. 69(4): p. 1442-50.
116. Kraemer, W.J., et al., Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int J Sports Med, 1991. 12(2): p. 228-35.
117. Kraemer, W.J., et al., Changes in hormonal concentrations after different heavy-resistance exercise protocols in women. J Appl Physiol, 1993. 75(2): p. 594-604.